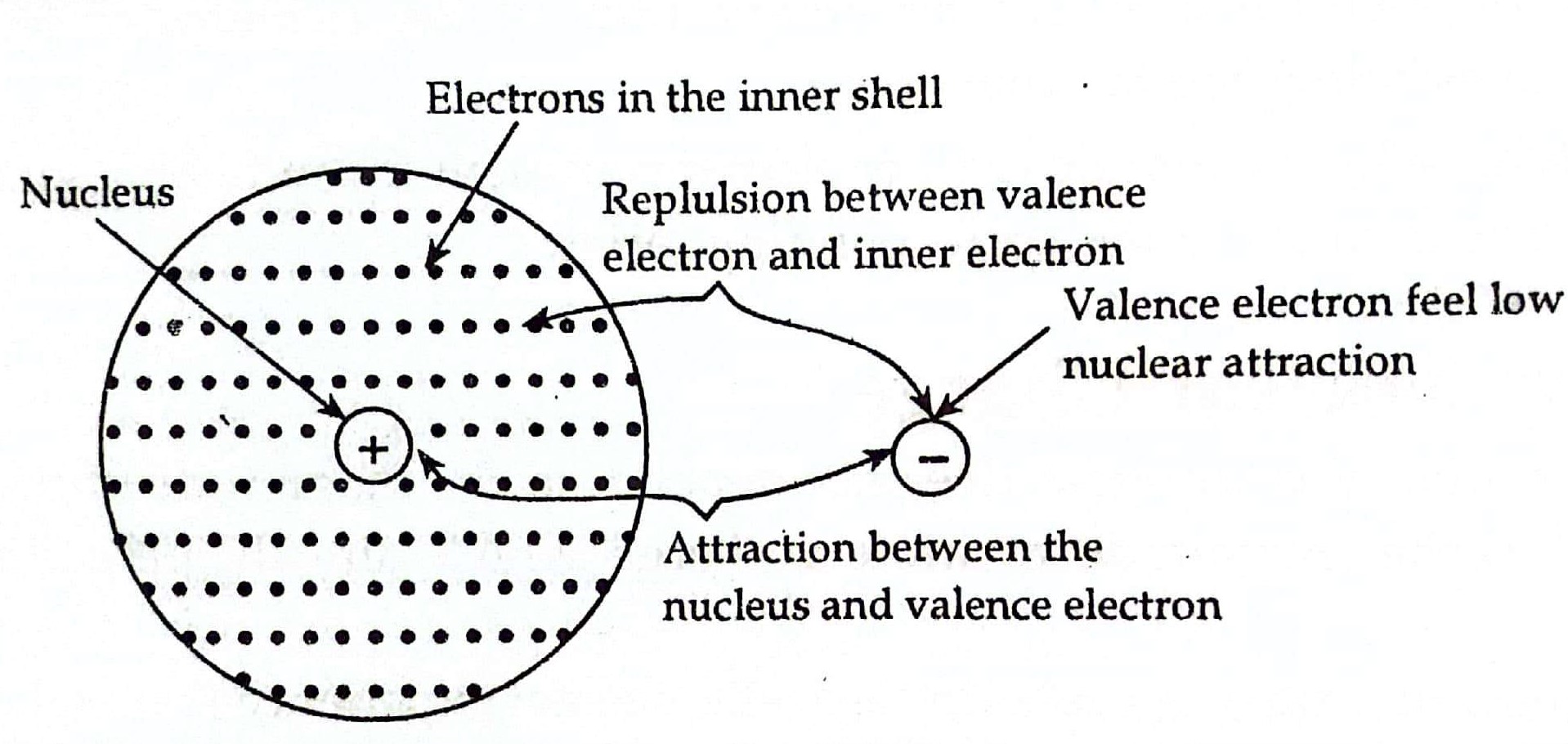
What Is Electron Shielding Mean . the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. S shell an s shell is a spherical shaped. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron.
from chemistnotes.com
the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron.
Shielding Effect or Screening Effect Definition, Factors Affecting
What Is Electron Shielding Mean the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen.
From www.nuclear-power.com
Shielding of Beta Radiation Types & Uses What Is Electron Shielding Mean the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive. What Is Electron Shielding Mean.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with. What Is Electron Shielding Mean.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Electron Shielding Mean S shell an s shell is a spherical shaped. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Unit 5 The Periodic Table PowerPoint Presentation, free download What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. the concept of electron shielding, in which intervening electrons act. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra What Is Electron Shielding Mean the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. shielding is the effect of inner shell. What Is Electron Shielding Mean.
From www.nuclear-power.com
Shielding of Neutron Radiation Types & Uses What Is Electron Shielding Mean conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. S shell an s shell is a spherical shaped. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. The nucleus of an atom contains protons, which carry positive charges,. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. The nucleus of an atom contains protons, which carry positive charges,. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Electron Shielding Mean The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act. What Is Electron Shielding Mean.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e What Is Electron Shielding Mean The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding,. What Is Electron Shielding Mean.
From www.slideserve.com
PPT Notes One Unit Nine Chapter Four PowerPoint Presentation, free What Is Electron Shielding Mean The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. conceptually, this means that d and f electrons are shielded 100% by all electrons. What Is Electron Shielding Mean.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. conceptually, this means that d and f electrons are shielded 100% by. What Is Electron Shielding Mean.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is Electron Shielding Mean S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of. What Is Electron Shielding Mean.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Electron Shielding Mean S shell an s shell is a spherical shaped. conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. What Is Electron Shielding Mean.
From www.youtube.com
nuclear charge and electron shielding YouTube What Is Electron Shielding Mean S shell an s shell is a spherical shaped. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use of hydrogen. the concept of electron shielding,. What Is Electron Shielding Mean.
From www.youtube.com
Chapter 3 Shielding Effect and Atomic Size YouTube What Is Electron Shielding Mean conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. S shell an s shell is a spherical shaped. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. What Is Electron Shielding Mean.
From www.elevate.in
Shielding Why And How? EPR, 51 OFF What Is Electron Shielding Mean conceptually, this means that d and f electrons are shielded 100% by all electrons in the same shell with a smaller value of \(l\), as well as. S shell an s shell is a spherical shaped. shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. The nucleus. What Is Electron Shielding Mean.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is Electron Shielding Mean shielding is the effect of inner shell electrons close to the nucleus reducing the nuclear charge on the valence electron. S shell an s shell is a spherical shaped. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in energy. the concept of electron shielding, in which intervening electrons act. What Is Electron Shielding Mean.